Demystifying Anion Gap
Serum Anion gap is very cofusing topic. In this article, the concept of anion gap is simplified to make it easy to understand.
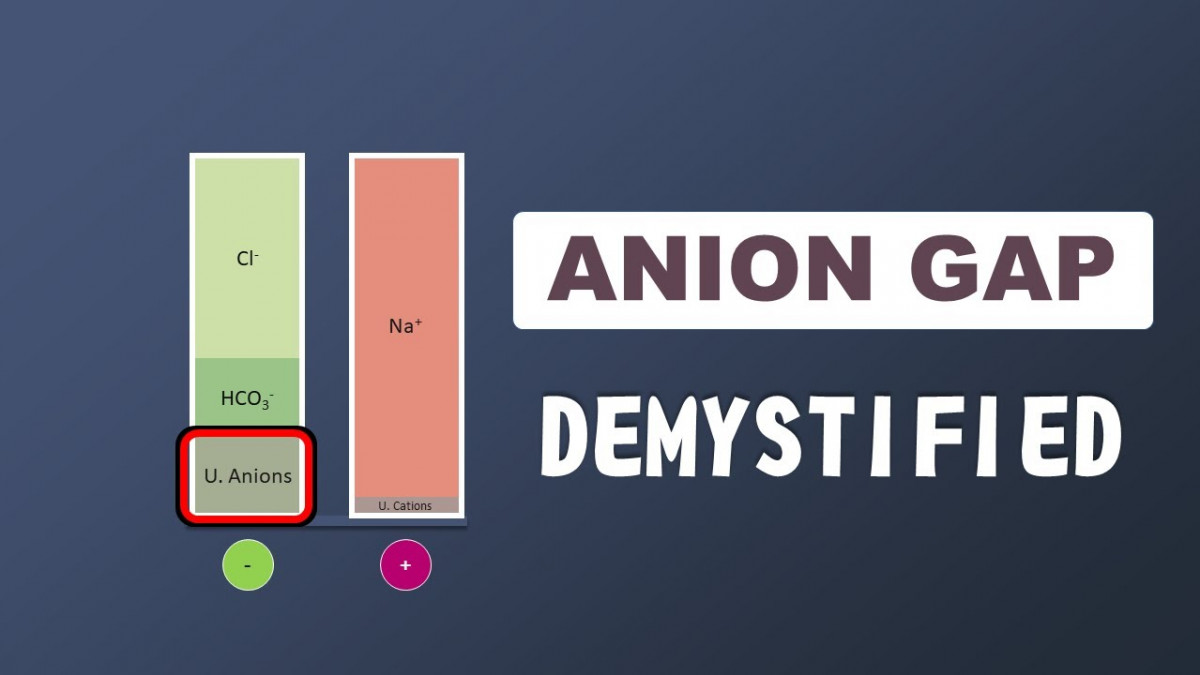
Anion Gap
If you are here, chances are you must have seen this equation perhaps more than once in your lifetime:
ANION GAP = [Na+] + [K+] - [Cl-] - [HCO3-]
Along with a Table mentioning causes of Increase Anion Gap and Normal Anion Gap.
You must be wondering where this equation came from and what it really means. And how come the anion gap is increasing or is normal in certain conditions. Today we will discuss all this in a very easy and logical way.
Let start the discussion with a very simple question:
Plasma has a net negative charge, positive charge or it is electrically neutral?
It is Neutral

So, it means to maintain the electrical neutrality the total
number of negatively charged ions must be equal to the total
number of positively charged ions in our plasma.
In an electrical field positively charged ion move towards cathode, so they are called cations while negatively charged ions move towards the anode, so they are called anions
So,
Total Anions = Total Cations

Some cations are routinely measured in the laboratory while some other that are not routinely measured in the laboratory. The same is true for anions.
So,
Measured Anions + Unmeasured Anions = Measured Cations + Unmeasured Cations
Subtracting “Unmeasured Cations” from both sides of the above equation
Measured Anions + Unmeasured Anions - Unmeasured Cations = Measured Cations + Unmeasured Cations - Unmeasure Cations
Measured Anions + Unmeasured Anions - Unmeasured Cations = Measured Cations
Subtracting Measured Anions from both sides of the above equation
Measured Anions + Unmeasured Anions- Unmeasured Cations - Measured Anions = Measured Cations - Measured Anions
Unmeasured Anions - Unmeasured Cations = Measured Cations - Measured Anions
Measured cations are Na+ and K+ and Measure Anions are Cl- and HCO3-, So the above equation becomes:
Unmeasured Anions - Unmeasured Cations= ([Na+] + [K+]) - ([Cl-] + [HCO3-])
Unmeasured Anions - Unmeasured Cations= [Na+] + [K+] - [Cl-] - [HCO3-]
Note: Some authors don’t consider K+ in the above equation.
If you look carefully, this above equation is actually the equation of the Anion Gap. So,
Anion gap = Unmeasured Anions - Unmeasured Cations= [Na+] + [K+] - [Cl-] - [HCO3-]
So, we can conclude from above the equation that Anion gap is basically mathematically calculated difference between Unmeasured Anions and Unmeasured cations
Unmeasured Cations are very small quantitatively and are not much relevant in the discussion of acid-base balance. Let’s ignore it.
Anion gap ≅ Unmeasured Anions≅ [Na+] + [K+] - [Cl-] - [HCO3-]
So, we can put this in another way that
“Anion gap is a mathematical calculation of Unmeasured Anions in our Plasma”
So what constitutes unmeasured anions in our plasma.
They could be:
- Phosphate ion
- Sulfate ion
- Negatively charged Plasma proteins (Albumin for example)
- Conjugate bases of Organic acids (I will explain this point later)
If we solve the equation of Anion gap:
Anion gap ≅ Unmeasured Anions ≅ 140 + 4 - 108 - 24
Anion gap ≅ Unmeasured Anions ≅ 12mg/dl
Anion gap varies according to Lab and whether or not Potassium is considered.
Generally, it ranges from 8-18mg/dl
Now that we have clearly understood what the Anion gap is. Where this equation came from and what does it represent? Now, we should address the question that what is its significance? What is it used for?
You know Bicarbonate ion is the alkali reserve of our body. It is the base fighting part of a major buffer of the plasma. When an acid is added into plasma it will neutralize the acid.

Let’s suppose you are exercising for too long, Glucose is
being converted into Pyruvic acid to generate energy/ATP, Pyruvic acid further undergoes TCA cycle to generate more energy in the presence of oxygen. But when you are exercising for too long, oxygen is insufficient for the TCA cycle. So as the TCA cycle slows down, Pyruvic Acid starts to accumulate and is converted into Lactic acid.
Lactic acid accumulates which causes muscle cramps. Lactic acid can also accumulate in the blood, due to tissue hypoxia. For example, Cardiac failure or shock. This accumulation of lactic acid is called Lactic Acidosis
Lactic acid consists of Lactate attached with a Proton (Lactate--H).
When it is released in an aqueous environment such as Plasma. It will donate the Proton (H+) while itself becoming a negatively charged conjugate base (Lactate-). Remember, Acid is a substance that donates protons in an aqueous environment.
This Proton is actually the culprit that imparts acidic character to anything.
Acidity depends upon the concentration of Free protons in a solution. As we had seen in the lecture on the concept of pH that; greater the number of free protons in a solution, lower will be its pH and vice versa.
So, Bicarbonate ion will come into play to neutralize these Free protons up to a certain limit. Bicarbonate will react with the proton and will be converted to H2CO3 (Carbonic acid). Bicarbonate which was a negatively charged; measured anion is lost during this reaction. (H2CO3 electrically neutral).
As more and more lactic acid accumulates, more and more Bicarbonate start to lose, I mean Measured Anion is decreasing. Also, because bicarbonate is lost, and pH is decreasing as Lactic acid continues to accumulate. This type of acidosis by definition is Metabolic Acidosis.
Now because Bicarbonate, the measured anion is decreasing, it means the total number of negatively charged ions is lost. So plasma might have become positively charge or you can say positive charge overpowers. So electrical neutrality may be lost. But this does not happen.
If you look carefully, not just a measured anion is lost, but a negatively charged anion is added into plasma as well. What is this negatively charged anion? Its Lactate, in this case. The conjugate base of Lactic acid, an organic acid.
Do we routinely measure the lactate levels in plasma? Of course not? So it is an unmeasured anion. So, this kind of metabolic acidosis in which unmeasured anion is increasing is called Increased Anion Gap Metabolic Acidosis.
Can you guess another cause of Increased Anion Gap Metabolic Acidosis?
Any type of poisoning leads to the generation of organic acid inside our body.
For example Salicylic acid poisoning (Aspirin).
Salicylic Acid, as discussed in the case of Lactic acidosis, will be dissociated into Salicylate and Proton. Proton will use up Bicarbonate and Salicylate ion will increase the anion gap.
Other examples:
- Ethanol poising because it is metabolized in our body and is converted into Formic acid. Which is an organic acid.
- Ethylene glycol is used as a radiator coolant agent. Its poising can lead to increased anion gap metabolic acidosis as it will generate Glycolic acid
- Ketoacidosis:
- In a severe shortage of Glucose (Starvation), or when the body fails to utilize glucose (Extremely low Insulin level), the body generates an alternate fuel for the body that is called ketone bodies. Ketone bodies are acidic substances and are also called Ketoacids. Their accumulation in starvation or severe uncontrolled Diabetes mellitus is called Ketoacidosis
- Ketoacids will, in a similar fashion, donate a proton, leaving behind their conjugate base that will increase the anion gap.
Now, this was about Increased Anion Gap Metabolic Acidosis. In some cases of Metabolic Acidosis Anion Gap Remain Normal. These are called Normal Anion Gap Metabolic Acidosis.
How can the anion gap remain normal in metabolic acidosis?
The answer is when unmeasured anions are not elevated. For example, when Bicarbonate is lost without a concurrent increase in unmeasured anions.
How can this happen?
Bicarbonate can be lost from two places.
1. GIT (Small intestine)
2. Kidneys
You know small intestines are abundant in bicarbonate, because acidic chyme that is released into the small intestine from the pylorus of the stomach, can be damaging for the small intestinal epithelial cells. Furthermore, digestive enzymes in the small intestine require an alkaline medium. So Bicarbonate ion is here to maintain this high pH in the small intestine.

When severe and prolonged diarrhea occurs, bicarbonate is lost. Losing Bicarbonate, the alkali reserve of our body means there will be metabolic acidosis. But in this type of metabolic acidosis as there is no concurrent increase in the unmeasured anion.
An overall net positive charge may ensue. So who will come into play to prevent this? In this case, Chloride will increase. Chloride may come out of cells, or it may be reabsorbed more from GIT or from Kidneys to compensate for the loss of negative charge.
As chloride is increasing and unmeasured anion remains the same, Anion gap will be normal. (Anion gap is a mathematical calculation of unmeasured anions).
Such type of Acidosis is the Normal Anion gap Metabolic Acidosis or Hyperchloremic metabolic acidosis. Naturally, the other type of metabolic acidosis in which anion gap increases is called Normochloremic Metabolic Acidosis.
Increased Bicarbonate loss from Kidneys will also cause normal anion gap metabolic acidosis. It can occur due to a problem in renal tubules and this is called Renal Tubular Acidosis. It is of different types, I am not going to tell you the details here.
Carbonic Anhydrase inhibitors like Acetazolamide can also cause loss of Bicarbonate in urine and will lead to normal anion gap metabolic acidosis.

Aldosterone hormone causes increase Sodium reabsorption and increase Potassium secretion from distal tubules of the nephrons. When Aldosterone is deficient due to any disease or lowered by drugs, Na+ will increase and K+ level will rise in plasma.
Hyperkalemia (increased potassium) will stimulate K+/H+ exchanger in cells of the body thus normalizing the Potassium level to some extent along with increasing in proton into plasma thus causing metabolic acidosis.
Such exchangers are widely present in the intestine and kidneys and cause loss of potassium at the expense of the gain of protons.
This type of acidosis is called Hyperkalemic Hyperchloremic metabolic acidosis.
Anion gap will be normal because unmeasured anion is not increasing. Chloride will increase to compensate somewhat. Also because Sodium (Measured Cations) level is low -> Anion gap will remain normal.
Aldosterone may be lowered when there is a problem with the adrenal gland. Adrenal Gland fails to Add Adrenal cortical hormones, a condition called Addison’s disease. All Adrenal cortical hormones including aldosterone will be deficient in this disease.

Angiotensin II is a stimulator of the release of Aldosterone from the Adrenal gland. Angiotensin-Converting Enzyme inhibitors or blockers can cause aldosterone deficiency. So overdose of ACEI though uncommonly can cause such type of normal anion gap / hyperkalemic metabolic acidosis.
In summary
Metabolic acidosis is of two types based on Anion Gap.
1. Increased Anion Gap Metabolic Acidosis
2. Normal Anion Gap Metabolic Acidosis

Sulfate and Phosphates are continuously being produced in our body or being ingested, furthermore all the time we produce acid. Its the responsibility of the kidney to get rid of these substances. So if Kidney failure is there, Sulphate and Phosphates will accumulate thus leading to an increase in unmeasured anion and raising anion gap along with that accumulation of protein will cause Metabolic acidosis.
So chronic kidney failure is another cause of Increased Anion Gap Metabolic Acidosis.
Significance of Anion Gap
- Classify metabolic acidosis based on Anion gap
- Increased Anion Gap Metabolic Acidosis
- Normal Anion Gap Metabolic Acidosis
- Diagnosis of certain conditions based on Anion gap
- Quality Control of Lab results
- If a normal healthy person’s measured ions yield an abnormal anion gap it may indicate faulty lab equipment or technique.
- Diagnosis of certain conditions based on Anion gap
606cac7498488_lg.png)
It is very difficult to explain the above-mentioned causes of increased and decreased anion gap in text form. Please watch the video lecture for an explanation.
 Dr. Aizaz
Dr. Aizaz 